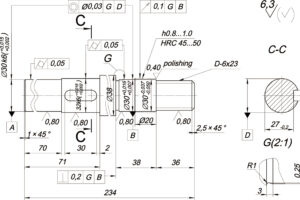
Electroplating is the process of plating a thin layer of other metals or alloys on the surface of certain metals using the principle of electrolysis. Improve wear resistance, electrical conductivity, reflectivity, corrosion resistance, and enhance aesthetics. The outer layers of many coins are also electroplated.
How does electroplating work?
The technology of depositing well-adhered metal coatings on mechanical products using the electrolytic cell principle, but with different properties and substrate materials. The electroplating layer is more uniform than the hot-dip layer and is generally thinner, ranging from several microns to tens of microns. Through electroplating, decorative protective and various functional surface layers can be obtained on mechanical products, and parts worn and machined incorrectly can also be repaired.
What are the electroplating methods?
Electroplating is divided into rack plating, barrel plating, continuous plating, and brush plating, which are mainly related to the size and batch of the parts to be plated. Rack plating is suitable for general-sized products, such as car bumpers, bicycle handlebars, etc. Barrel plating is ideal for small parts such as fasteners, washers, pins, etc. A continuous plate is suitable for mass-produced wire and strip. Brush plating is perfect for partial scale or repair. The electroplating solution includes acid, alkaline, and acid and neutral solutions with chromium compounds. No matter what plating method is used, the plating tank, hanger, etc., that are in contact with the product to be plated and the plating solution should have a particular general purpose.
There are also different functions according to various electroplating needs. Can be divided into:
- Copper plating: used as a primer to improve the adhesion and corrosion resistance of the electroplating layer. (Copper is easy to oxidize. After oxidation, the patina is no longer conductive, so copper must protect copper-plated products)
- Nickel plating: used as a primer or as an appearance to improve corrosion resistance and wear resistance (among which chemical nickel is the modern process with more wear resistance than chrome plating). (Note that many electronic products, such as DIN head and N head, no longer use nickel as the base, mainly because nickel is magnetic, which will affect the passive intermodulation in the electrical performance)
- Gold plating: Improve conductive contact resistance and enhance signal transmission. (Gold is the most stable and most expensive.)
- Palladium-nickel plating: Improves conductive contact resistance, improves signal transmission, and has higher wear resistance than gold.
- Tin and lead plating: improve the welding ability and will be replaced by other substitutes soon (because most of the lead is now plated with bright tin and matte tin).
- Silver plating: Improve conductive contact resistance and enhance signal transmission. (Silver has the best performance, easy to oxidize, and also conducts electricity after oxidation)
What is the coating classification?
According to the composition of the coating, it can be divided into three types: single metal coating, alloy coating, and composite coating.
If classified by use, it can be divided into:
- protective coating;
- protective decorative coating;
- decorative coating;
- repair coating;
- functional coating
What is single metal plating?
Single-metal electroplating has a history of more than 170 years, and there are 33 metals on the periodic table that can be prepared by electrodeposition from aqueous solutions. Commonly used are more than 10 kinds of electro-galvanized, nickel, chromium, copper, tin, iron, cobalt, cadmium, lead, gold, silver, and so on. The plating layer formed by simultaneously depositing two or more elements on the cathode is an alloy plating layer. The alloy coating has the structure and properties that a single metal coating does not have, such as amorphous Ni-P alloy, each core Sn alloy that is not on the phase diagram and has a unique decorative appearance, exceptionally high corrosion resistance, and excellent weldability. , Magnetic alloy coating, etc.
What is composite plating?
Composite plating is a process in which solid particles are added to the plating solution and co-deposited with metals or alloys to form a metal-based surface composite material to meet unique application requirements. According to the electrochemical properties between the coating and the base metal, the electroplating layer can be divided into two categories: anodic coating and cathodic coating. When the potential of the coating metal relative to the base metal is negative, the coating is an anode when forming a corrosion microbattery, so it is called an anodic coating, such as the galvanized layer on steel parts; and when the potential of the coating metal relative to the base metal is positive, When the corrosion micro battery is formed, the coating is the cathode, so it is called cathodic coatings, such as the nickel-plated layer and the tin-plated layer on the steel parts.
The classification can be divided into:
- Protective coating: coatings such as Zn, Ni, Cd, Sn, and Cd-Sn are used as anti-corrosion coatings for atmospheric and various corrosive environments;
- Protection, decorative coating: such as Cu-Ni-Cr, Ni-Fe-Cr composite coating, etc., both decorative and protective;
- Decorative coating: such as Au, Ag, and Cu. Sun imitation gold coating, black chrome, black nickel coating, etc.;
- Reparative coating: For example, electroplating Ni, Cr, and Fe layer to repair some high-cost wear parts or processing out-of-tolerance parts;
- Functional coatings: conductive coatings such as Ag and Au; magnetic conductive coatings such as Ni-Fe, Fe-Co, Ni-Co; high-temperature anti-oxidation coatings such as Cr and Pt-Ru; reflective coatings such as Ag and Cr; black chrome, Anti-reflective coatings such as black nickel; hard chrome, Ni. SiC and other wear-resistant coatings; Ni. VIEE, Ni. C (graphite) anti-friction coating, etc.; Pb, Cu, Sn, Ag, and other weldable coatings; anti-carburizing Cu coating, etc.